.png)
How Can Real-World Evidence Be Used to Expedite Long COVID Treatments?
Traditional research moves slowly, but patients with Long COVID can’t wait. Learn how Long COVID Labs is charting a new path to bring treatments closer, faster.
PATIENT #1 - 1 MONTH UPDATE
One month ago, Patient #1 in our case studies series received a dose of Pemgarda monoclonal antibodies through his doctor, under the EUA. He combined this with 10 days of Paxlovid, with the goal of clearing persistent SARS-CoV-2 virus, which we strongly believe is the root cause of Long COVID for many patients.
We know so many of you have been eager for updates - here’s the latest on this patient, and some responses to frequently asked questions.

On days 2-6 following his infusion, Patient #1 reported an 80% reduction in brain fog and fatigue, with an increase in PEM symptoms. Light exertion that was previously tolerable was now triggering a crash, although the Patient had significantly more energy and clarity of thought. On day 5, Patient stopped his 10 day Paxlovid course.
From day 7-9 following the infusion, the Patient’s brain fog and fatigue returned.
From day 9 onwards, the Patient’s PEM and POTS symptoms continued to increase in severity, dropping below his pre-treatment baseline. For the following 2 weeks the Patient experienced a worsening of symptoms that caused a reduction in mobility and lifestyle. Near day 30, the symptoms slowly returned back to baseline.
Now, one month later, Patient #1 is returning to his pre-treatment baseline. He has informed us that both the benefits as well as the negative side effects both appear to be diminishing, and he feels similarly to how he felt before the infusion.
At this point, it’s not completely clear why Patient #1 had the results that he did – and we recognize that much more data is needed.
That’s why we are adapting our approach to meet the challenges of this moment.

NEXT STEPS
Based on the success of our first case study, we’re excited to announce a shift in our strategy.
Since our initial launch, the landscape of Long COVID treatment has changed. When we first entered the space, it was rare to hear of patients accessing root-cause treatments - and our priority was to help fill in that gap.
Now, we’re happy to say that some things have changed for the better. We’ve been hearing more and more stories from patients across the world who’ve been accessing promising treatments.
Patients are moving faster than trials. But the outcomes aren’t being standardized and analyzed in a way that helps move the community or research forward.
On top of that, without biomarker tests like HRV or SARS-CoV-2 viral load, it's often unclear how certain treatments are working, and whether they’re just helping to manage a patient’s symptoms or actually targeting the root cause.
At Long COVID Labs, we believe these problems are highly solvable – and we’re looking at how to best position ourselves to create maximum impact.
EVOLVING OUR ROLE FROM OPERATOR TO INFRASTRUCTURE

We’re shifting our focus from individual case studies to building the infrastructure that captures, amplifies, and validates the real-world experimentation already happening inside clinics today.
We’ve started building a modern, smart Long COVID patient registry that collects and structures patient data (including symptoms, treatments, biomarkers and wearables). With permission from our participants, we’ll be sharing the anonymized data open-source. This means that any interested parties - whether that’s patients, researchers, or pharma companies - can look at our datasets and find information they can use, in a format that’s publishable in scientific journals.
We don’t want our data to sit in a vault - we want it to belong to the community and inspire change as rapidly as possible.
THE TREATMENT LEADERBOARD
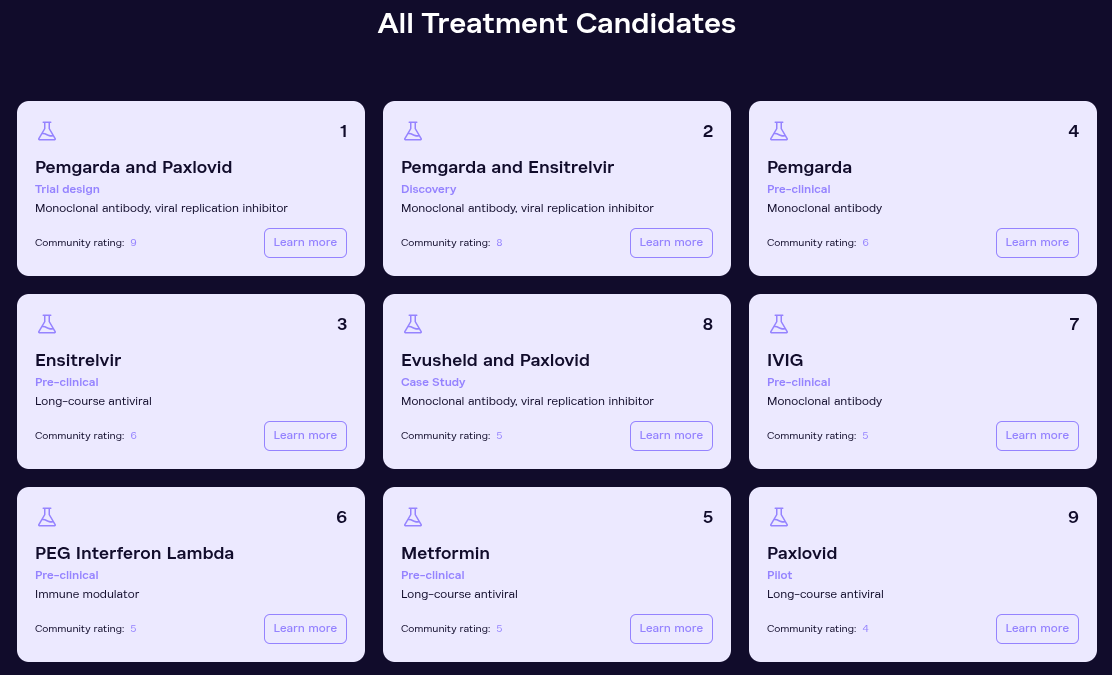
As we analyze the data we receive from patients and clinicians, we’ll be able to identify early signals of efficacy from the treatments patients are trying. With our platform, we can act as a megaphone for these patients and the doctors they’re working with, helping to draw pharmaceutical interest and potentially supporting clinical trials for the most promising candidates ourselves.
PATIENT GOVERNANCE
Patients who share their data may receive governance tokens that give them voting rights within the project. Patient contributors will be able to have a say in which treatment studies we prioritize, and how the intellectual property we create can be ethically used. We’re a non-profit operating for the public good - any profits we generate will be re-invested to fund our mission to accelerate a cure for Long COVID.

SHARE YOUR DATA WITH US!
If you have Long COVID, we’d love to have you fill out our Patient Symptom Survey and let us know about your experience and symptom profile.
Additionally, if you’re trying any experimental treatments, we’d love to have you fill out the survey before and after, and let us know how things are going!
If you have any questions, you can always comment or DM us on social media, or send an email to support@longcovidlabs.org.
Thank you so much for your support along the way! We can’t wait to hear from you.